Zinc–zinc oxide cycle
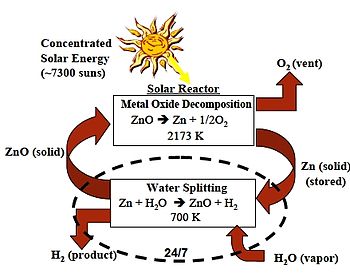
For chemical reactions, the zinc–zinc oxide cycle or Zn–ZnO cycle is a two step thermochemical cycle based on zinc and zinc oxide[1] for hydrogen production[2] with a typical efficiency around 40%.[3]
Process description
The thermochemical two-step water splitting process uses redox systems:[4]
- Dissociation: ZnO → Zn + 1/2 O2
- Hydrolysis: Zn + H2O → ZnO + H2
For the first endothermic step concentrating solar power is used in which zinc oxide is thermally dissociated at 1,900 °C (3,450 °F) into zinc and oxygen. In the second non-solar exothermic step zinc reacts at 427 °C (801 °F) with water and produces hydrogen and zinc oxide. The temperature level is realized by using a solar power tower and a set of heliostats to collect the solar thermal energy.
See also
- Cerium(IV) oxide–cerium(III) oxide cycle
- Copper–chlorine cycle
- Hydrosol-2
- Hybrid sulfur cycle
- Iron oxide cycle
- Sulfur–iodine cycle
References
- ^ Solar Hydrogen Production from a ZnO/Zn Thermo-chemical Cycle Archived July 24, 2008, at the Wayback Machine
- ^ Project PD10
- ^ Novel Method for solar hydrogen generation Archived February 5, 2009, at the Wayback Machine
- ^ Solar thermal ZnO-decomposition
External links
- H2 formation by zinc hydrolysis in a hot wall aerosol flow reactor








