| MET proto-oncogene, receptor tyrosine kinase |
|---|
|
| Identifikatori |
|---|
| Alijasi | |
|---|
| Spoljašnji ID | GeneCards: [1] |
|---|
| Obrazac RNK izražavanja |
|---|
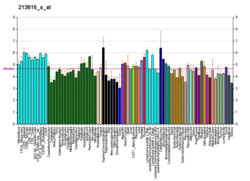 | | More reference expression data |
|
| Ortolozi |
|---|
| Vrste | Čovek | Miš |
|---|
| Entrez | | |
|---|
| Ensembl | | |
|---|
| UniProt | | |
|---|
| RefSeq (mRNA) | | |
|---|
| RefSeq (protein) | | |
|---|
| Location (UCSC) | n/a | n/a |
|---|
| PubMed search | n/a | n/a |
|---|
| Wikidata |
|
c-Met (MET, receptor hepatocitnog faktora rasta, HGFR),[1][2] protein je koji je kod ljudi kodiran MET genom (MET proto-onkogena receptorska tirozinska kinaza). U ranijem razdoblju istraživačkog procesa takođe je nazivan MNNG HOS transformišući gen. Ova protein deluje kao tirozinska kinaza.[3] Primarni jednolančani prekursorni protein se posttranslaciono preseca i time se formiraju alfa i beta podjedinica, koje se disulfidno vezuju pri formiraju krajnjeg oblika receptora.
MET je membranski receptor koji je esencijalan za embrionsko razviće i zarastanje rana. Hepatocitni faktor rasta (HGF) je jedini poznati ligand MET receptora. MET je normalno izražen u ćelijama epitelnog porekla, dok je izražavanje HGF ograničeno na ćelije mesenhimalnog porekla. Nakon HGF stimulacije, MET indukuje nekoliko bioloških responsa koji kolektivno formiraju program poznat kao invazivni rast.
Interakcije
Met formira interakcije sa:
Vidi još
- c-Met inhibitori
- Tpr-met fuzioni protein
Reference
- ^ Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, Stuart AA (1991). „Identification of the hepatocyte growth factor receptor as the met proto-oncogene product”. Science. 251 (4995): 802—4. PMID 1846706. doi:10.1126/science.1846706.
- ^ Galland F, Stefanova M, Lafage M, Birnbaum D (1992). „Localization of the 5' end of the MCF2 oncogene to human chromosome 15q15----q23”. Cytogenet. Cell Genet. 60 (2): 114—6. PMID 1611909. doi:10.1159/000133316.
- ^ Cooper CS (1992). „The met oncogene: from detection by transfection to transmembrane receptor for hepatocyte growth factor”. Oncogene. 7 (1): 3—7. PMID 1531516.
- ^ Davies G, Jiang WG, Mason MD (2001). „HGF/SF modifies the interaction between its receptor c-Met, and the E-cadherin/catenin complex in prostate cancer cells”. Int. J. Mol. Med. 7 (4): 385—8. PMID 11254878. doi:10.3892/ijmm.7.4.385.
- ^ Petrelli A, Gilestro GF, Lanzardo S, Comoglio PM, Migone N, Giordano S (2002). „The endophilin-CIN85-Cbl complex mediates ligand-dependent downregulation of c-Met”. Nature. 416 (6877): 187—90. PMID 11894096. doi:10.1038/416187a.
- ^ Ng C, Jackson RA, Buschdorf JP, Sun Q, Guy GR, Sivaraman J (2008). „Structural basis for a novel intrapeptidyl H-bond and reverse binding of c-Cbl-TKB domain substrates”. EMBO J. 27 (5): 804—16. PMC 2265755
 . PMID 18273061. doi:10.1038/emboj.2008.18.
. PMID 18273061. doi:10.1038/emboj.2008.18. - ^ Grisendi S, Chambraud B, Gout I, Comoglio PM, Crepaldi T (2001). „Ligand-regulated binding of FAP68 to the hepatocyte growth factor receptor”. J. Biol. Chem. 276 (49): 46632—8. PMID 11571281. doi:10.1074/jbc.M104323200.
- ^ Ponzetto C, Zhen Z, Audero E, Maina F, Bardelli A, Basile ML, Giordano S, Narsimhan R, Comoglio P (1996). „Specific uncoupling of GRB2 from the Met receptor. Differential effects on transformation and motility”. J. Biol. Chem. 271 (24): 14119—23. PMID 8662889. doi:10.1074/jbc.271.24.14119.
- ^ Liang Q, Mohan RR, Chen L, Wilson SE (1998). „Signaling by HGF and KGF in corneal epithelial cells: Ras/MAP kinase and Jak-STAT pathways”. Invest. Ophthalmol. Vis. Sci. 39 (8): 1329—38. PMID 9660480.
- ^ Comoglio PM (1993). „Structure, biosynthesis and biochemical properties of the HGF receptor in normal and malignant cells”. EXS. 65: 131—65. PMID 8380735.
- ^ Naldini L, Weidner KM, Vigna E, Gaudino G, Bardelli A, Ponzetto C, Narsimhan RP, Hartmann G, Zarnegar R, Michalopoulos GK (1991). „Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor”. EMBO J. 10 (10): 2867—78. PMC 452997
 . PMID 1655405.
. PMID 1655405. - ^ Hiscox S, Jiang WG (1999). „Association of the HGF/SF receptor, c-met, with the cell-surface adhesion molecule, E-cadherin, and catenins in human tumor cells”. Biochem. Biophys. Res. Commun. 261 (2): 406—11. PMID 10425198. doi:10.1006/bbrc.1999.1002.
- ^ Wang D, Li Z, Messing EM, Wu G (2002). „Activation of Ras/Erk pathway by a novel MET-interacting protein RanBPM”. J. Biol. Chem. 277 (39): 36216—22. PMID 12147692. doi:10.1074/jbc.M205111200.
Literatura
- Peruzzi B, Bottaro DP (2006). „Targeting the c-Met signaling pathway in cancer”. Clin. Cancer Res. 12 (12): 3657—60. PMID 16778093. doi:10.1158/1078-0432.CCR-06-0818.
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF (2003). „Met, metastasis, motility and more”. Nat. Rev. Mol. Cell Biol. 4 (12): 915—25. PMID 14685170. doi:10.1038/nrm1261.
- Zhang YW, Vande Woude GF (2003). „HGF/SF-met signaling in the control of branching morphogenesis and invasion”. J. Cell. Biochem. 88 (2): 408—17. PMID 12520544. doi:10.1002/jcb.10358.
- Paumelle R, Tulasne D, Kherrouche Z, Plaza S, Leroy C, Reveneau S, Vandenbunder B, Fafeur V, Tulashe D, Reveneau S (2002). „Hepatocyte growth factor/scatter factor activates the ETS1 transcription factor by a RAS-RAF-MEK-ERK signaling pathway”. Oncogene. 21 (15): 2309—19. PMID 11948414. doi:10.1038/sj.onc.1205297.
- Comoglio PM (1993). „Structure, biosynthesis and biochemical properties of the HGF receptor in normal and malignant cells”. EXS. 65: 131—65. PMID 8380735.
- Maulik G, Shrikhande A, Kijima T, Ma PC, Morrison PT, Salgia R (2002). „Role of the hepatocyte growth factor receptor, c-Met, in oncogenesis and potential for therapeutic inhibition”. Cytokine Growth Factor Rev. 13 (1): 41—59. PMID 11750879. doi:10.1016/S1359-6101(01)00029-6.
- Ma PC, Maulik G, Christensen J, Salgia R (2003). „c-Met: structure, functions and potential for therapeutic inhibition”. Cancer Metastasis Rev. 22 (4): 309—25. PMID 12884908. doi:10.1023/A:1023768811842.
- Knudsen BS, Edlund M (2004). „Prostate cancer and the met hepatocyte growth factor receptor”. Adv. Cancer Res. Advances in Cancer Research. 91: 31—67. ISBN 978-0-12-006691-9. PMID 15327888. doi:10.1016/S0065-230X(04)91002-0.
- Dharmawardana PG, Giubellino A, Bottaro DP (2004). „Hereditary papillary renal carcinoma type I”. Curr. Mol. Med. 4 (8): 855—68. PMID 15579033. doi:10.2174/1566524043359674.
- Kemp LE, Mulloy B, Gherardi E (2006). „Signalling by HGF/SF and Met: the role of heparan sulphate co-receptors”. Biochem. Soc. Trans. 34 (Pt 3): 414—7. PMID 16709175. doi:10.1042/BST0340414.
Spoljašnje veze
- Proto-Oncogene+Proteins+c-met на US National Library of Medicine Medical Subject Headings (MeSH)
- UniProtKB/Swiss-Prot entry P08581: MET_HUMAN, ExPASy (Expert Protein Analysis System) proteomics server of the Swiss Institute of Bioinformatics (SIB)
- A table with references to significant roles of MET in cancer
-
1r0p: Kristalna struktura tirozinskog kinaznog domena receptora hepatocitnog faktora rasta, C-MET, u kompleksu sa mikrobnim alkaloidom K-252a -
1r1w: Kristalna struktura tirozinskog kinaznog domena receptora hepatocitnog faktora rasta, C-MET -
1shy: Kristalna struktura HGF beta-lanca u kompleksu sa Sema domenom Met receptora. -
1ssl: Kristalna struktura PSI domena Met receptora -
2g15: Strukturna karakterizacija autoinhibirane c-Met kinaze |
|
|---|
|
|---|
| EGF receptorska familija | |
|---|
| Insulin receptor familija | |
|---|
| PDGF receptor familija | CSF1R • FLT3 • KIT • PDGFR (PDGFRA, PDGFRB) |
|---|
| FGF receptor familija | FGFR1 • FGFR2 • FGFR3 • FGFR4 |
|---|
| VEGF receptor familija | VEGFR1 • VEGFR2 • VEGFR3 • VEGFR4 |
|---|
| HGF receptor familija | MET • RON |
|---|
| Trk receptor familija | |
|---|
| EPH receptor familija | EPHA1 • EPHA2 • EPHA3 • EPHA4 • EPHA5 • EPHA6 • EPHA7 • EPHA8 • EPHB1 • EPHB2 • EPHB3 • EPHB4 • EPHB5 • EPHB6 • EPHX |
|---|
| LTK receptor familija | LTK • ALK |
|---|
| TIE receptor familija | TIE • TEK |
|---|
| ROR receptor familija | ROR1 • ROR2 |
|---|
| DDR receptor familija | |
|---|
| PTK7 receptor familija | |
|---|
| RYK receptor familija | RYK |
|---|
| MuSK receptor familija | |
|---|
| ROS receptor familija | ROS1 |
|---|
| AATYK receptor familija | AATYK • AATYK2 • AATYK3 |
|---|
| AXL receptor familija | AXL • MER • TYRO3 |
|---|
| RET receptor familija | RET |
|---|
| nekategorisani | STYK1 |
|---|
|
|
|
|---|
| ABL familija | ABL1 • ARG |
|---|
| ACK familija | ACK1 • TNK1 |
|---|
| CSK familija | CSK • MATK |
|---|
| FAK familija | FAK • PYK2 |
|---|
| FES familija | FES • FER |
|---|
| FRK familija | FRK • BRK • SRMS |
|---|
| JAK familija | JAK1 • JAK2 • JAK3 • TYK2 |
|---|
| SRC-A familija | SRC • FGR • FYN • YES1 |
|---|
| SRC-B familija | BLK • HCK • LCK • LYN |
|---|
| TEC familija | TEC • BMX • BTK • ITK • TXK |
|---|
| SYK familija | SYK • ZAP70 |
|---|
|
|
B enzm: 1.1/2/3/4/5/6/7/8/10/11/13/14/15-18, 2.1/2/3/4/5/6/7/8, 2.7.10, 2.7.11-12, 3.1/2/3/4/5/6/7, 3.1.3.48, 3.4.21/22/23/24, 4.1/2/3/4/5/6, 5.1/2/3/4/99, 6.1-3/4/5-6 |
|
|---|
| Nervni faktori rasta | niski afinitet/p75 – visok afinitet Trk ( TrkA, TrkB, TrkC) – Cilijarni neurotrofni faktor |
|---|
| Somatomedin | |
|---|
| CSF | |
|---|
| TGF put | TGF-beta (1, 2) – Aktivin (1, 2) – Bone morfogenetski protein (1, 2) |
|---|
| Drugi | |
|---|
vidi isto Faktori rasta |
 . PMID 18273061. doi:10.1038/emboj.2008.18.
. PMID 18273061. doi:10.1038/emboj.2008.18.  . PMID 1655405.
. PMID 1655405.  1r0p: Kristalna struktura tirozinskog kinaznog domena receptora hepatocitnog faktora rasta, C-MET, u kompleksu sa mikrobnim alkaloidom K-252a
1r0p: Kristalna struktura tirozinskog kinaznog domena receptora hepatocitnog faktora rasta, C-MET, u kompleksu sa mikrobnim alkaloidom K-252a 1r1w: Kristalna struktura tirozinskog kinaznog domena receptora hepatocitnog faktora rasta, C-MET
1r1w: Kristalna struktura tirozinskog kinaznog domena receptora hepatocitnog faktora rasta, C-MET 1shy: Kristalna struktura HGF beta-lanca u kompleksu sa Sema domenom Met receptora.
1shy: Kristalna struktura HGF beta-lanca u kompleksu sa Sema domenom Met receptora. 1ssl: Kristalna struktura PSI domena Met receptora
1ssl: Kristalna struktura PSI domena Met receptora 2g15: Strukturna karakterizacija autoinhibirane c-Met kinaze
2g15: Strukturna karakterizacija autoinhibirane c-Met kinaze















