UBE2G2
| UBE2G2 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | UBE2G2, UBC7, ubiquitin conjugating enzyme E2 G2 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 603124; MGI: 1343188; HomoloGene: 6599; GeneCards: UBE2G2; OMA:UBE2G2 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Ubiquitin-conjugating enzyme E2 G2 is a protein that in humans is encoded by the UBE2G2 gene.[5][6][7]
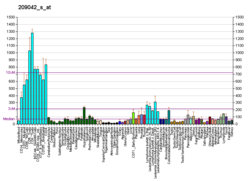
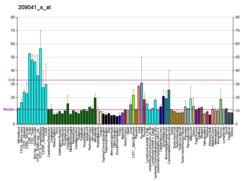
The modification of proteins with ubiquitin is an important cellular mechanism for targeting abnormal or short-lived proteins for degradation. Ubiquitination involves at least three classes of enzymes: ubiquitin-activating enzymes, or E1s, ubiquin-conjugating enzymes, or E2s, and ubiquitin-protein ligases, or E3s. This gene encodes a member of the E2 ubiquitin-conjugating enzyme family. The encoded protein shares 100% sequence identity with the mouse counterpart. This gene is ubiquitously expressed, with high expression seen in adult muscle. Two alternatively spliced transcript variants encoding distinct isoforms have been found for this gene.[7] Ube2g2 is known to interact with a variety of other proteins, including ubiquitin, the AMFR (E3 gp78), and the SYVN1 (Hrd1 RING).
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000184787 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000009293 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Katsanis N, Fisher EM (Sep 1998). "Identification, expression, and chromosomal localization of ubiquitin conjugating enzyme 7 (UBE2G2), a human homologue of the Saccharomyces cerevisiae ubc7 gene". Genomics. 51 (1): 128–31. doi:10.1006/geno.1998.5263. PMID 9693041.
- ^ Rose SA, Leek JP, Moynihan TP, Ardley HC, Markham AF, Robinson PA (Mar 1999). "Assignment1 of the ubiquitin conjugating enzyme gene, UBE2G2, to human chromosome band 21q22.3 by in situ hybridization". Cytogenet Cell Genet. 83 (1–2): 98–9. doi:10.1159/000015141. PMID 9925943. S2CID 13129608.
- ^ a b "Entrez Gene: UBE2G2 ubiquitin-conjugating enzyme E2G 2 (UBC7 homolog, yeast)".
Further reading
- Chen P, Johnson P, Sommer T, et al. (1993). "Multiple ubiquitin-conjugating enzymes participate in the in vivo degradation of the yeast MAT alpha 2 repressor". Cell. 74 (2): 357–69. doi:10.1016/0092-8674(93)90426-Q. PMID 8393731. S2CID 205020910.
- Moynihan TP, Ardley HC, Nuber U, et al. (1999). "The ubiquitin-conjugating enzymes UbcH7 and UbcH8 interact with RING finger/IBR motif-containing domains of HHARI and H7-AP1". J. Biol. Chem. 274 (43): 30963–8. doi:10.1074/jbc.274.43.30963. PMID 10521492.
- Huang L, Kinnucan E, Wang G, et al. (1999). "Structure of an E6AP-UbcH7 complex: insights into ubiquitination by the E2-E3 enzyme cascade". Science. 286 (5443): 1321–6. doi:10.1126/science.286.5443.1321. PMID 10558980.
- Hattori M, Fujiyama A, Taylor TD, et al. (2000). "The DNA sequence of human chromosome 21". Nature. 405 (6784): 311–9. Bibcode:2000Natur.405..311H. doi:10.1038/35012518. PMID 10830953.
- Lenk U, Sommer T (2001). "Ubiquitin-mediated proteolysis of a short-lived regulatory protein depends on its cellular localization". J. Biol. Chem. 275 (50): 39403–10. doi:10.1074/jbc.M006949200. PMID 10991948.
- Joazeiro CA, Hunter T (2000). "Biochemistry. Ubiquitination--more than two to tango". Science. 289 (5487): 2061–2. doi:10.1126/science.289.5487.2061. PMID 11032556. S2CID 83324199.
- Tiwari S, Weissman AM (2001). "Endoplasmic reticulum (ER)-associated degradation of T cell receptor subunits. Involvement of ER-associated ubiquitin-conjugating enzymes (E2s)". J. Biol. Chem. 276 (19): 16193–200. doi:10.1074/jbc.M007640200. PMID 11278356.
- Fang S, Ferrone M, Yang C, et al. (2002). "The tumor autocrine motility factor receptor, gp78, is a ubiquitin protein ligase implicated in degradation from the endoplasmic reticulum". Proc. Natl. Acad. Sci. U.S.A. 98 (25): 14422–7. doi:10.1073/pnas.251401598. PMC 64697. PMID 11724934.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. Bibcode:2002PNAS...9916899M. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Webster JM, Tiwari S, Weissman AM, Wojcikiewicz RJ (2003). "Inositol 1,4,5-trisphosphate receptor ubiquitination is mediated by mammalian Ubc7, a component of the endoplasmic reticulum-associated degradation pathway, and is inhibited by chelation of intracellular Zn2+". J. Biol. Chem. 278 (40): 38238–46. doi:10.1074/jbc.M305600200. PMID 12869571.
- Ota T, Suzuki Y, Nishikawa T, et al. (2004). "Complete sequencing and characterization of 21,243 full-length human cDNAs". Nat. Genet. 36 (1): 40–5. doi:10.1038/ng1285. PMID 14702039.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The Status, Quality, and Expansion of the NIH Full-Length cDNA Project: The Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Rual JF, Venkatesan K, Hao T, et al. (2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature. 437 (7062): 1173–8. Bibcode:2005Natur.437.1173R. doi:10.1038/nature04209. PMID 16189514. S2CID 4427026.
- Chen B, Mariano J, Tsai YC, et al. (2006). "The activity of a human endoplasmic reticulum-associated degradation E3, gp78, requires its Cue domain, RING finger, and an E2-binding site". Proc. Natl. Acad. Sci. U.S.A. 103 (2): 341–6. Bibcode:2006PNAS..103..341C. doi:10.1073/pnas.0506618103. PMC 1326157. PMID 16407162.
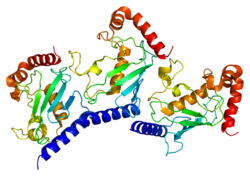
- Arai R, Yoshikawa S, Murayama K, et al. (2006). "Structure of human ubiquitin-conjugating enzyme E2 G2 (UBE2G2/UBC7)". Acta Crystallographica Section F. 62 (Pt 4): 330–4. doi:10.1107/S1744309106009006. PMC 2222581. PMID 16582478.
- Hu YH, Warnatz HJ, Vanhecke D, et al. (2006). "Cell array-based intracellular localization screening reveals novel functional features of human chromosome 21 proteins". BMC Genomics. 7: 155. doi:10.1186/1471-2164-7-155. PMC 1526728. PMID 16780588.
- Ewing RM, Chu P, Elisma F, et al. (2007). "Large-scale mapping of human protein–protein interactions by mass spectrometry". Mol. Syst. Biol. 3 (1): 89. doi:10.1038/msb4100134. PMC 1847948. PMID 17353931.
External links
- Overview of all the structural information available in the PDB for UniProt: P60604 (Ubiquitin-conjugating enzyme E2 G2) at the PDBe-KB.
- v
- t
- e
-
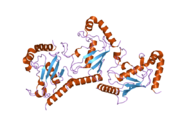 2cyx: Structure of human ubiquitin-conjugating enzyme E2 G2 (UBE2G2/UBC7)
2cyx: Structure of human ubiquitin-conjugating enzyme E2 G2 (UBE2G2/UBC7)
 | This article on a gene on human chromosome 21 is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e